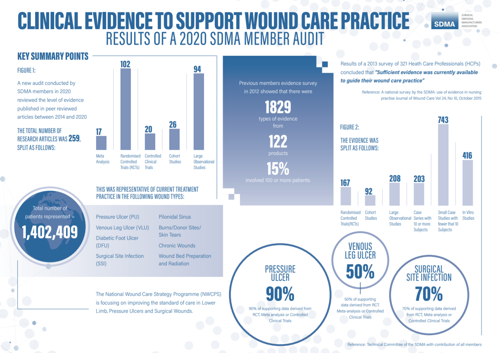
CLINICAL EVIDENCE TO SUPPORT WOUND CARE PRACTICE
Topic : Type : Graphic
There is an opinion held by some wound care stakeholders that clinical evidence for wound care products is lacking.
However, a 2020 SDMA audit indicates that 259 clinical studies were conducted over six years, representing over 1.4 million patients, suggesting that there is sufficient evidence to guide clinical practice.
The over emphasis on the primacy of the RCT in the hierarchy of evidence and decision making has long been debated. Many healthcare professionals recognise that real world data also provides valuable evidence, as the heterogeneity and variability in wound care outcomes mean that these studies may provide realistic and relevant measures on dressings performance. The increase in supported self-management during the COVID-19 pandemic highlight that both the patient’s and clinicians views should be considered and reflected.
There is also increased acknowledgement from leading Health Technology Assessment bodies such as NICE of the need to use real-world data to resolve gaps in knowledge and drive forward access to innovations for patients (NICE 5 year strategy).
The SDMA and ABHI are calling for discussion between Health Technology Assessment bodies, the National Wound Care Strategy Programme, wound care practitioners and industry to arrive at an agreed level of evidence to help guide best clinical practice to support existing and future innovation adoption across the wound care arena.
An infographic that provides further detail on this call, can be viewed below.
Click on the infographic to download it.